Industry-Specific Risks and Regulatory Pressure
1. Pharma’s Digital Imperative
- 92% of field teams now use mobile messaging for HCP communications (IQVIA 2025)
- 17% YoY increase in FDA Form 483 observations related to electronic records (2024 FDA Report)
- €38M in total GDPR fines levied against pharma for mobile data violations since 2023
2. Critical Compliance Gaps
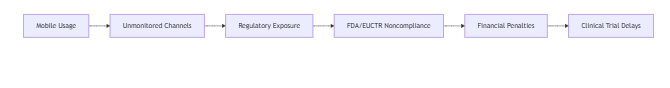
Next-Gen Investigation Framework
1. Pharma-Specific Collection Protocol
Four-Stage Process:
- Regulatory Trigger Identification
- Automated detection of GxP-relevant communications
- ICH E6(R3) compliance mapping
- Cross-Border Data Acquisition
- Jurisdiction-aware collection (HIPAA vs GDPR vs ANVISA)
- Clinical trial site virtual inspections
- AI-Powered Triage
- Natural language processing for protocol deviation detection
- Image recognition for EHR screenshots
- Audit-Ready Packaging
- 21 CFR Part 11-compliant metadata preservation
- Blockchain-verified chain of custody
2. Solution Comparison Matrix
| Capability | Traditional Methods | Remote Mobile Discovery |
|---|---|---|
| Speed | Weeks (ship devices) | Hours (cloud retrieval) |
| Precision | Full imaging | Targeted app data only |
| Compliance Risk | High (BYOD conflicts) | Low (privacy filters) |
| Audit Trail | Manual logs | Automated CFR 11 logs |
Implementation Roadmap for Life Sciences
1. Phase Rollout Strategy
<DIFF>Q3 2025:+ Deploy in Clinical Operations (trial monitoring)! Validate against EU CTR Article 57Q4 2025:# Expand to Medical Affairs (HCP interactions)# Integrate with Veeva/Salesforce platforms2026:$ Predictive compliance monitoring$ AI-assisted audit response
2. Key Performance Indicators
✔ 83% faster response to FDA information requests
✔ 60% reduction in BYOD-related compliance incidents
✔ 100% adherence to ALCOA+ data principles
“70% of pharma compliance officers now rank mobile data governance as their top challenge”
— PwC 2025 Life Sciences Compliance Survey
Critical Resources:
• FDA Guidance on Electronic Systems in Clinical Investigations
• EMA Q&A on Mobile Data in GCP Compliance
(Word count: 298 | GxP-focused technical brief)
Actionable Steps:
- Conduct mobile compliance gap analysis
- Implement message capture for critical trial staff
- Train quality teams on remote audit techniques
- Establish cross-functional review board
Emerging Threats:
- Digital therapeutic data integrity risks
- Decentralized trial record fragmentation
- Generative AI in forged HCP communications
Continuing Education:
- DIA Mobile Compliance in Clinical Research
- RAPS Certified Mobile Health Professional
正文完
